Positive electrode material
High performance positive electrode material
Spherical nickel hydroxide with a diameter of about 10μm, which has a high filling property, is used as the positive electrode material for nickel-metal hydride batteries.
Cobalt hydroxide is generally used in the positive electrode as the conductive material, and as shown in the figure, it dissolves in an alkaline electrolyte and coats the surface of nickel hydroxide.
Cobalt hydroxide is oxidized on the first charge and changes to highly conductive cobalt oxyhydroxide, which plays a role in increasing the conductivity of the electrode.
Cobalt hydroxide is generally used in the positive electrode as the conductive material, and as shown in the figure, it dissolves in an alkaline electrolyte and coats the surface of nickel hydroxide.
Cobalt hydroxide is oxidized on the first charge and changes to highly conductive cobalt oxyhydroxide, which plays a role in increasing the conductivity of the electrode.
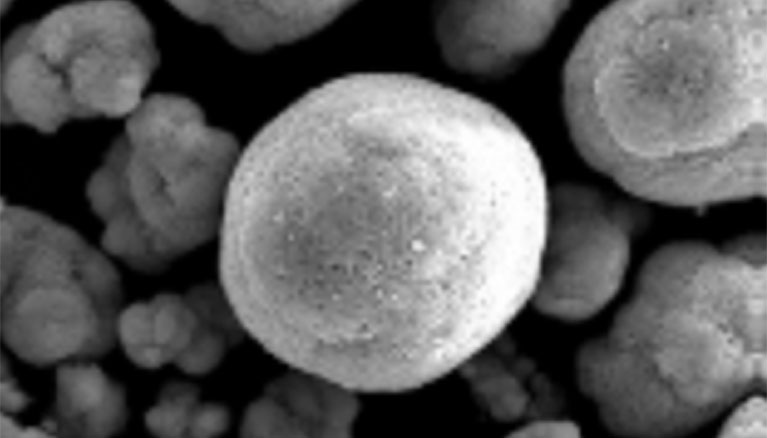
Spherical nickel hydroxide

Nickel hydroxide
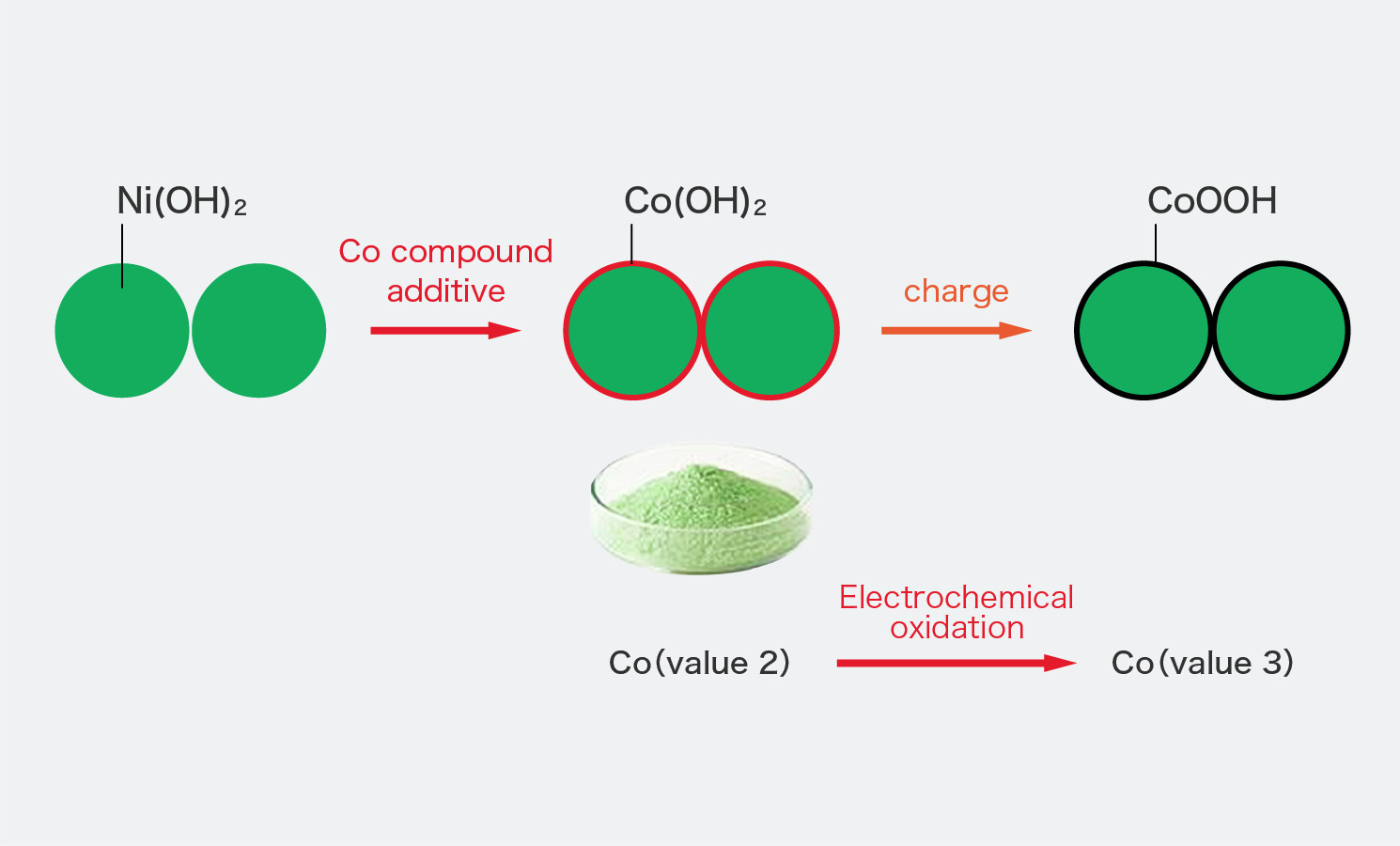
Oxidation of cobalt compounds in batteries
FDK's original technology
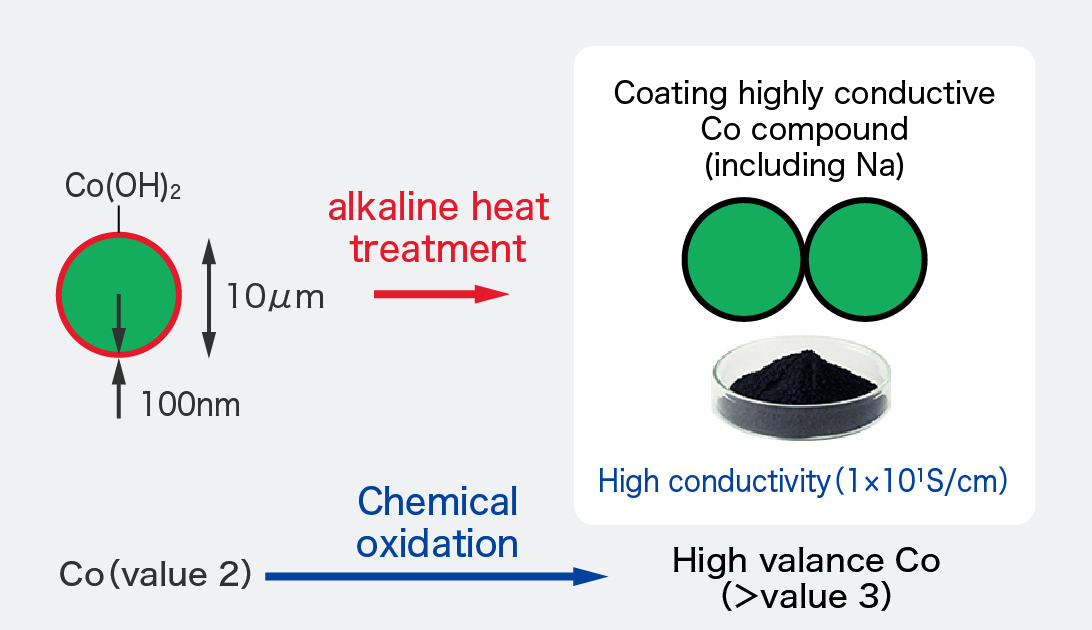
As shown in the figure, FDK uses nickel hydroxide coated with cobalt hydroxide, which is chemically oxidized with a highly conductive cobalt compound, as the positive electrode material.
The features of our original active material are as follows.
The features of our original active material are as follows.
- A special crystal structure that incorporates Na from chemical oxidation into the crystal structure
- High valence cobalt, that has excellent conductivity and durability
Cells using high conductivity cobalt compound coated nickel hydroxide have high performance such as a high capacity and high durability.
Introduction of sample analysis results at FDK
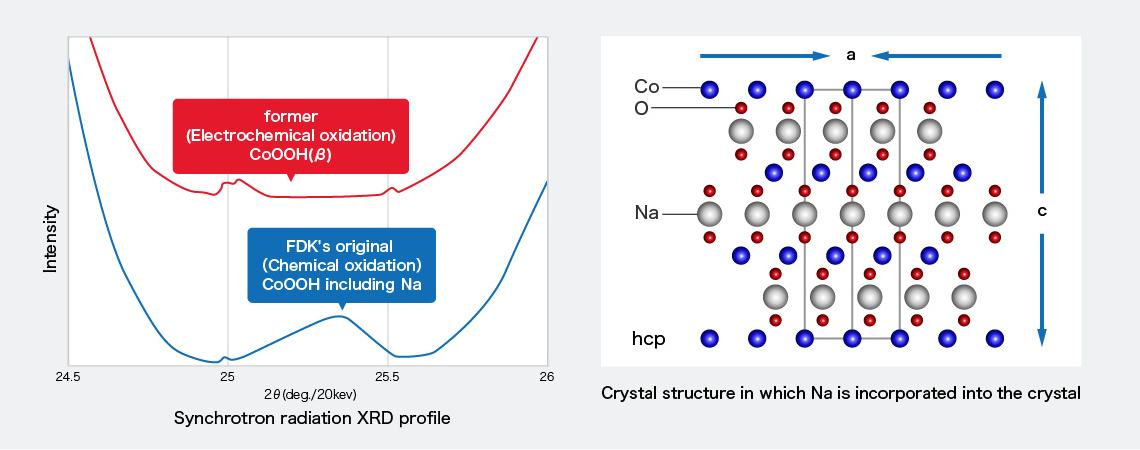
We have established the analytical material crystal structure techniques in cooperation with Fujitsu Laboratories Co., Ltd., using a synchrotron radiation X-ray device such as SPring8, a scanning electron microscope (SEM) and transmission electron microscope (TEM).From these analysis results, it was confirmed that the chemically oxidized cobalt oxyhydroxide has a higher cobalt valence than the electrochemically oxidized cobalt oxyhydroxide and has a specific crystal structure containing Na. *1
※1:ECS Transactions, 66 (8) 19-27 (2015)
Results of valence analysis of surface Co compound layer by synchrotron radiation XAFS analysis
| Oxidization method | Co value coating on nickel hydroxide | Conductive | ||
|---|---|---|---|---|
| After electrode manufacturing | After cell charge and discharge | After cell charge and discharge | ||
| FDK | Chemical oxidation | 3.15 | 3.22 | 1x10-1/Ω・㎝ |
| Co compound additive (Competitor) | Electrochemical oxidation | 2.00 | 2.97 | 1x10-4/Ω・㎝ |
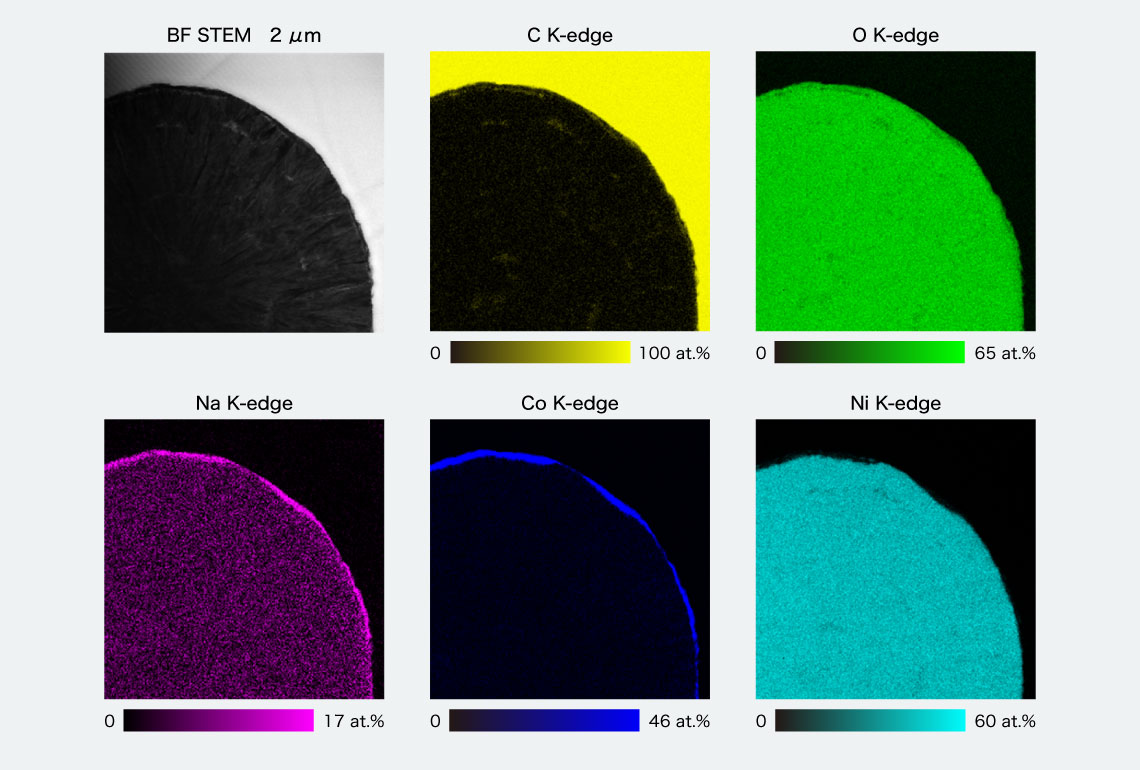
STEM image and EDX analysis of the surface area of nickel hydroxide coated with cobalt compound
We confirmed that the cobalt compound was uniformly coated with a layer in which Na and Co coexisted on the surface area of nickel hydroxide.
Crystal structure analysis of coated cobalt compounds using synchrotron radiation XRD
It was confirmed that different peaks were generated in the XRD profile because the c-axis length was extended and the a-axis length was contracted because Na was incorporated into the crystal, and the crystal structure was different from a conventional one.